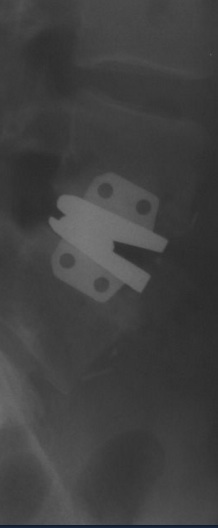
Image is courtesy of: "The Artificial Lumbar Disc".
Maverick Total Disc Replacement
Maverick is the first metal-on-metal, two-piece anterior lumbar disc prosthesis. Maverick attempts to relieve pain by stabilizing the diseased level without immobilizing it. Not one of the two models of stabilization device currently approved for use in the US, the Maverick TDR is a U.S. FDA Investigational Device Exemptions study device. Its US trial began in 2003.
Risks
- No long-term studies exist to confirm safety and longevity
- Poor diagnostics, imaging and/or preparation
- Inexperienced surgeon
- Insufficient disc space
- Canal encroachment/nerve injury
- Epidural bleeding
- Improper size selection or device placement
Benefits
- Mininal wear debris without compromising shock absorbtion
- Intended to improve motion in the treated vertebral body while sparing the facets from additional loading
- Easily inserted
- Promotes rapid ingrowth
- Long-lasting